- Development Boards
- DC
- Sensors
- Hall Effect Sensor
- touch switch
- Vibration Sensor
- Temperature sensor
- Photoresistor
- MQ Sensor Series
- Color sensor
- water level sensor
- stepper motor
- Buzzer
- Inclinometer
- Accelerometer
- Current & Voltage
- Infrared
- Flame
- Ultrasonic
- Pressure & Altitude
- Temperature & Humidity
- Color,Light,lmage
- Shake,Force,Bend
- Identity
- GPS & Location
- Biometric
- IMU,AHRS
- Contact
- Pulse Oximeter and Heart Rate Sensor
- Motion
- Touch
- Pressure and Temperature
- Gas Sensor
- Modules
- PWM Speed Controller
- Joystick Module
- music module
- Amplifier Board
- CLOCK
- Ultrasonic Ranging Module
- USB TO TTL
- Boost Board
- Conversion Module
- Serial port conversion module
- Power Amplifier Board
- CAN Bus
- Microphone Module
- Vibration Motor
- Data Acquisition
- NFC
- button
- rotary encoder
- Camera
- blue tooth
- Motor drive
- Displays
- Wireless & loT
- Power & Batteries
- Robotics
- Tools & Equipments
- Components
Table of Contents
Battery Safety in Public
Nowadays, batteries of various types—such as lithium batteries and sodium batteries—are widely used in our daily lives. Lithium batteries, in particular, power almost everything: the smartphones we carry in our pockets, the laptops we use for work, and the shared power banks we rent when out. On a larger scale, they also provide continuous energy for new energy buses in cities, emergency lights in subway stations, backup power cabinets in shopping malls, and even portable ECG monitors used for emergency care in hospitals. As for sodium batteries, due to their low cost and resistance to low temperatures, they have recently begun to be used in applications like energy storage systems.
However, battery safety incidents are truly disturbing. There have been cases where a lithium battery in a smartphone swelled and exploded after overcharging, even setting fire to the bedside cabinet. Some residents brought lithium batteries of electric bikes home to charge; at night, the batteries triggered thermal runaway due to overcharging, causing a fire in the early morning. Not only was their bedroom completely burned, but the fire also spread to the corridor, injuring neighbors who inhaled excessive smoke. Earlier, someone left an electric bike outdoors (when the temperature was as low as -20°C) and charged it with a fast charger. In just one hour, lithium dendrites formed inside the battery, leading to a short circuit and fire—three electric bikes and parts of the corridor walls were completely burned. From these incidents, you can surely see that battery safety is no trivial matter; it is closely tied to the safety of your family. Whether it is a household energy storage battery in the living room or an electric bike battery used for daily commuting, neglecting safety in any link may cause fires, electric shocks, and endanger lives and property.

Why are batteries prone to problems?
First, let’s talk about lithium batteries. Available in types like ternary lithium (nickel-cobalt-manganese lithium) and lithium iron phosphate (LFP), they are the first choice for consumer electronics and new energy vehicles. Their energy density is high, ranging from 180 to 300 Wh/kg, but this high energy is a double-edged sword—once out of control, the risks it releases are far greater than those of ordinary batteries.
Thermal runaway in lithium batteries is extremely dangerous; it is a chemical chain reaction where “one wrong step leads to a chain of errors.” For example, during overcharging (when the voltage of a single cell exceeds 4.3V), or when an electric vehicle collision crushes the battery, or when a foreign object pierces the battery pack, cathode materials like nickel-cobalt-manganese lithium will release oxygen rapidly. This oxygen reacts with carbonate-based substances in the electrolyte, generating a huge amount of heat instantly—local temperatures can soar above 800°C, and flammable gases such as methane and carbon monoxide are released. If these gases cannot escape, the battery will swell, catch fire, or even explode in severe cases.
Moreover, most lithium batteries are used in configurations where multiple cells are connected in series or parallel. However, even cells from the same production batch have inherent differences: capacity may vary by ±5%, internal resistance by ±10 mΩ, and voltage by ±0.02V. During use, these differences widen due to uneven charging and inconsistent local heat dissipation. For instance, during charging, cells with smaller capacity reach the cut-off voltage first. Without the control of a Battery Management System (BMS), the charger will continue to supply power, causing these cells to overcharge. During discharging, these same low-capacity cells reach the discharge cut-off voltage first, but the device still requires power, leading to over-discharging. Over time, these overused cells age rapidly, potentially swelling or leaking, and eventually become a trigger for the entire battery pack to malfunction.
As for sodium batteries, their maximum allowable discharge current is lower than that of lithium batteries. However, some devices require instantaneous high power—such as when a community electric patrol car accelerates or when an energy storage cabinet responds to sudden peak electricity demand. If the current exceeds the sodium battery’s safety threshold at such times, the internal resistance of the cell will increase sharply, local temperatures can rise above 60°C, and electrode materials will age quickly. In the end, the battery will either fail to charge or experience sudden discharge drops, making it unusable.

How to protect your batteries?
- Firmly avoid purchasing three-no batteries (no brand, no production date, no quality certification) and chargers; prioritize original accessories, as they offer better compatibility and safety guarantees.
- Do not modify batteries privately, and do not leave batteries in high-temperature environments (such as in a car trunk in summer) or humid environments for a long time. Extreme environments will accelerate battery damage.
- For large-capacity batteries like e-bike and energy storage batteries, try to charge them in outdoor dedicated charging areas during the daytime.
- Check the battery appearance once a month. If you find battery swelling, leakage, or cracked/loose casings, replace it immediately.
- Pay more attention to changes in battery performance. For example, if a mobile phone suddenly loses power quickly, it is likely that the battery is aging or there is an internal fault.
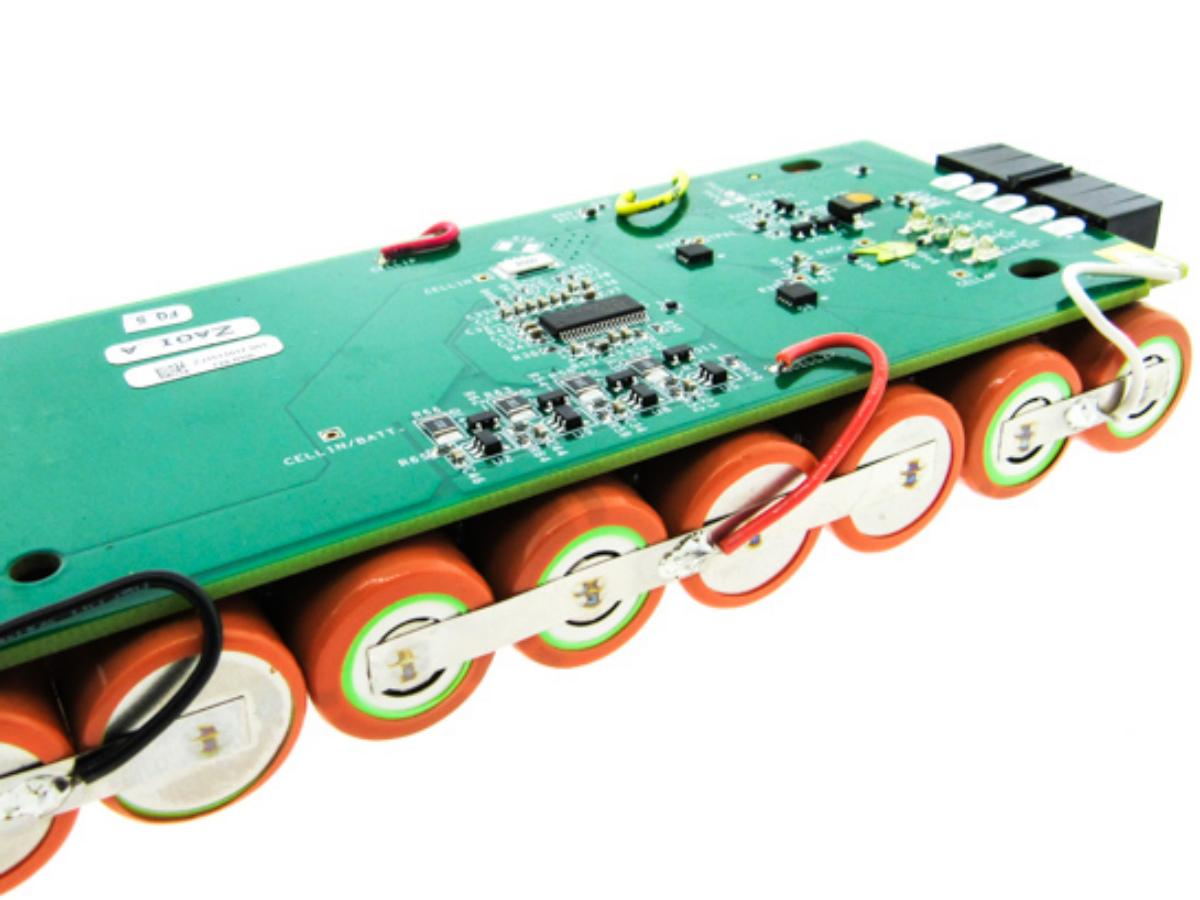
- Install a qualified BMS for the battery, which is the key to ensuring battery safety and performance.
Can you run a lithium/sodium battery without a BMS?
Some people may ask: Can lithium or sodium batteries be used without a BMS? To be honest, only in a handful of low-risk scenarios can they be used temporarily; in 99% of formal usage scenarios, a BMS is absolutely indispensable.
Multi-cell lithium battery packs—such as those in electric vehicle battery packs, laptop batteries, and energy storage batteries above 12V—are completely dependent on a BMS. For example, if 100 ternary lithium cells in an electric vehicle are connected in series without a BMS, the battery could catch fire due to overcharging of individual cells in just 5 minutes of charging. During discharging, voltage differences between cells can cause sudden power cuts, damaging the device. Only a few exceptions exist: single-cell, low-capacity lithium batteries (e.g., 1.5V button batteries or small toy batteries with a capacity below 500 mAh). Their low energy and current allow basic protection through simple fuses or zener diodes.
The same applies to multi-cell sodium battery systems. For example, household energy storage battery packs above 5 kWh must be equipped with a BMS. Take an energy storage cabinet with 10 sodium cells connected in series: without a BMS, after 100 charge-discharge cycles, the difference in cell capacity will widen by 15%, and the usable capacity will be reduced by half. Furthermore, sodium dendrites are likely to form during low-temperature charging, leaving a risk of short circuits. As for single-cell, low-power sodium batteries (e.g., 3.2V/1000 mAh batteries for small LED lights), they can be used without a BMS for a short time, but their lifespan will be significantly shortened.

What benefits of Battery Management Systems?
The core function of a BMS is to build a safety barrier for batteries, safeguarding the bottom line of personal and property safety. During battery use, it is inevitable to encounter four safety threats: overcharging, over-discharging, overcurrent, and extreme temperatures. Once out of control, these threats can cause minor issues like swelling/leakage or major disasters like fires/explosions—and the BMS is the only component that can resolve these risks in real time.
For example, when charging a lithium battery, once the voltage of a single cell reaches the safe cut-off value of 4.2V, the BMS will immediately cut off the charging circuit to prevent cathode material collapse and electrolyte decomposition, which would trigger thermal runaway. During discharging, if the voltage of a single cell drops to the critical value of 2.5V, the BMS will promptly cut off the discharge to avoid irreversible damage (such as lithium plating on the anode) or internal short circuits. In case of a short circuit or sudden high current caused by instantaneous high-power discharge—once the current exceeds the safety threshold set based on battery capacity—the overcurrent protection module in the BMS will interrupt the circuit instantly to prevent internal overheating and combustion. When exposed to high or low temperatures, the BMS monitors conditions in real time via temperature sensors: in high temperatures, it either activates the cooling system (e.g., the water cooling system in electric vehicles) or cuts off power directly to slow battery aging and avoid thermal runaway; in low temperatures, it actively limits charge-discharge power—this may reduce the range of electric vehicles in winter, but fundamentally prevents battery damage caused by lithium plating.
Beyond ensuring safety, the BMS also optimizes battery performance for a better user experience. Many people think that a battery’s actual capacity, charge-discharge speed, and range stability depend solely on the cells, but this is not the case. When multiple cells are connected in series or parallel (e.g., the battery pack with hundreds of cells in an electric vehicle), differences in capacity and voltage between cells are inevitable. This often leads to some cells being fully charged first or drained first, ultimately limiting the capacity of the entire battery pack. The BMS’s cell balancing function—whether active or passive—uses current compensation to keep all cells in a consistent state. This avoids waste such as a nominal 100 Ah battery only delivering 80 Ah of usable capacity, ensuring the battery can be fully charged and discharged to a safe minimum.
During charging, the BMS adjusts the current flexibly based on the state of charge (SOC) and temperature: it uses high-current fast charging (e.g., DC fast charging for electric vehicles) when the battery is low, and switches to trickle charging when the battery is nearly full. This ensures fast charging efficiency while preventing battery damage from overcharging. During discharging, it adjusts the output current according to load demands—for example, supplying more power when an electric vehicle accelerates (requiring high power) and providing stable power during steady-speed driving. Additionally, the BMS adapts to the needs of different devices: the BMS for energy storage stations adjusts charge-discharge rhythms based on grid load (charging during off-peak hours and discharging during peak hours to achieve peak-valley arbitrage); the BMS for smartphones automatically reduces screen brightness when the battery level drops below 20%, balancing battery life and device performance.
More importantly, the BMS significantly extends battery lifespan, reducing long-term usage costs—a crucial advantage for devices where batteries account for a large portion of costs (e.g., electric vehicles and energy storage stations). Battery lifespan is generally measured by the number of cycles until its capacity drops below 80%, which is directly affected by charge-discharge depth, temperature, and current. The BMS precisely controls these factors to prolong battery life. For example, it actively limits charge-discharge depth: electric vehicle batteries are usually not charged to 100% (some models default to 95%) and are not discharged to 0%—a low-battery alert is triggered when the level drops below 10%. Experimental data shows that the lifespan of lithium batteries cycled between 0% and 80% SOC is 2–3 times longer than that cycled between 0% and 100%. At the same time, the BMS prohibits charging and discharging in harsh conditions: it limits charging current when temperatures are below 0°C (to prevent lithium plating) and pauses charge-discharge when temperatures exceed 45°C (to avoid electrolyte decomposition and reduce irreversible cell damage). Furthermore, the BMS calculates the battery’s state of health (SOH) in real time. When the SOH drops below 80%, it further reduces charge-discharge power and narrows the charge-discharge range to slow capacity decay. For electric vehicles, battery replacement costs typically account for 30%–50% of the vehicle price; if the BMS can extend battery life by 1–2 years, it can save significant replacement and maintenance costs, lowering overall usage costs.
In addition, the BMS serves as an information bridge between the battery, the user, and the device. It collects key battery parameters in real time—including SOC, SOH, voltage, temperature, and current. These data are transmitted to the device’s control system and then displayed to users via an interface, allowing users to clearly understand the battery’s status.
One point must be emphasized: Battery systems without a BMS cannot meet mandatory industry standards or obtain market access qualifications—this cannot be ignored. When purchasing battery-related products, be sure to check if they are equipped with a qualified BMS, as this directly affects safety and user experience.
In short, without a BMS, batteries face direct risks of fire and explosion due to overcharging, over-discharging, and overcurrent. Multi-cell batteries will experience rapid capacity decay and significantly reduced charge-discharge efficiency. Users will have no way of knowing the battery’s remaining power or health status, and devices will be unable to adapt to different usage scenarios. Therefore, whether using lithium batteries or sodium batteries, installing a qualified BMS is extremely important.